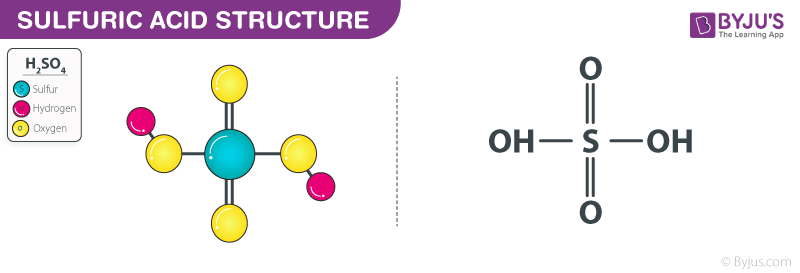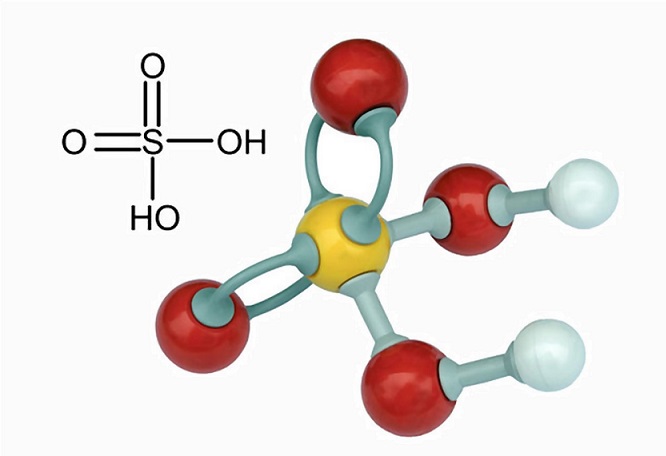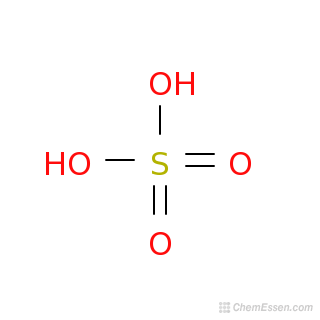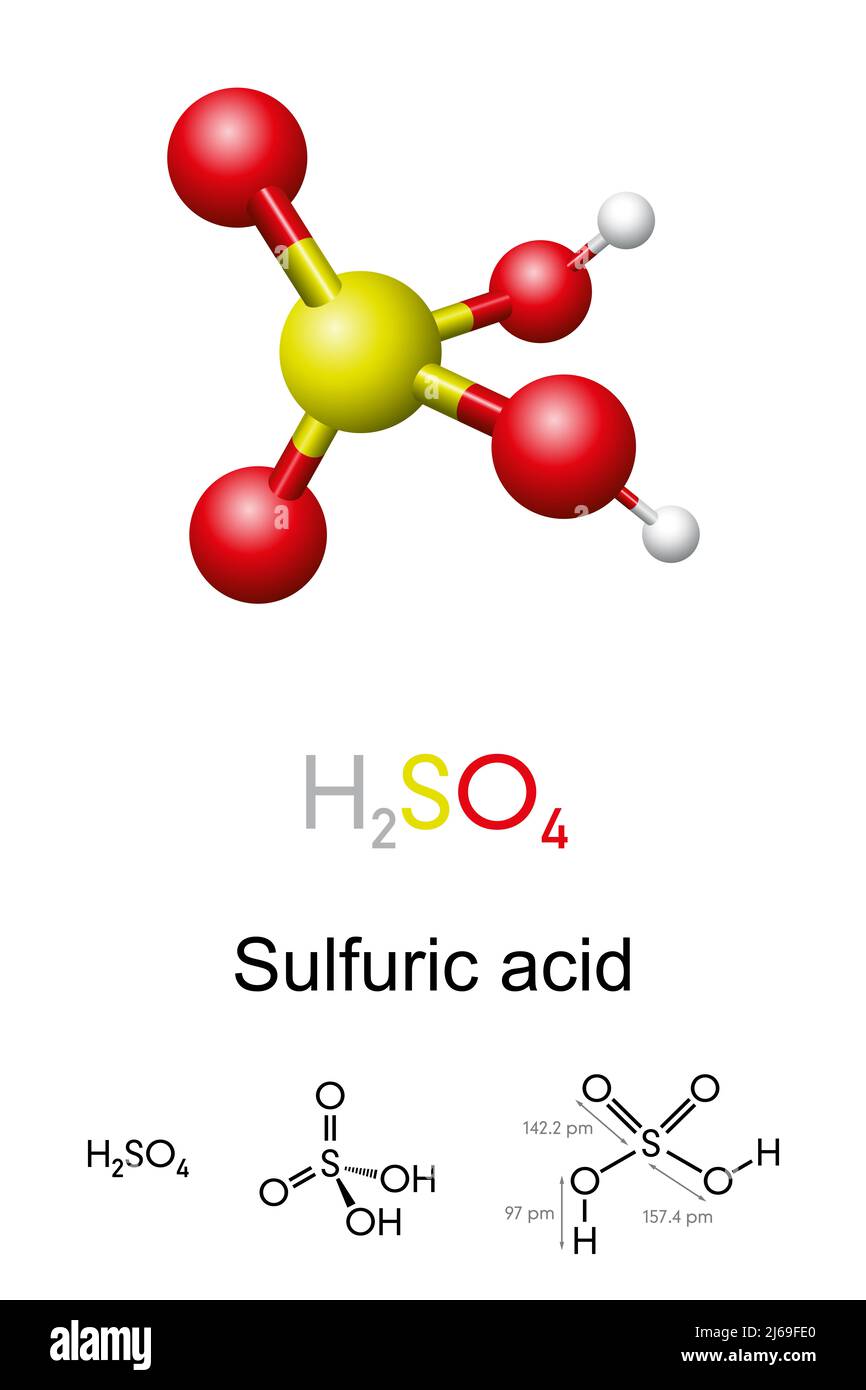
Sulfuric acid, H2SO4, ball-and-stick model, molecular and chemical formula with binding lengths. Known as sulphuric acid, or oil of vitriol Stock Photo - Alamy

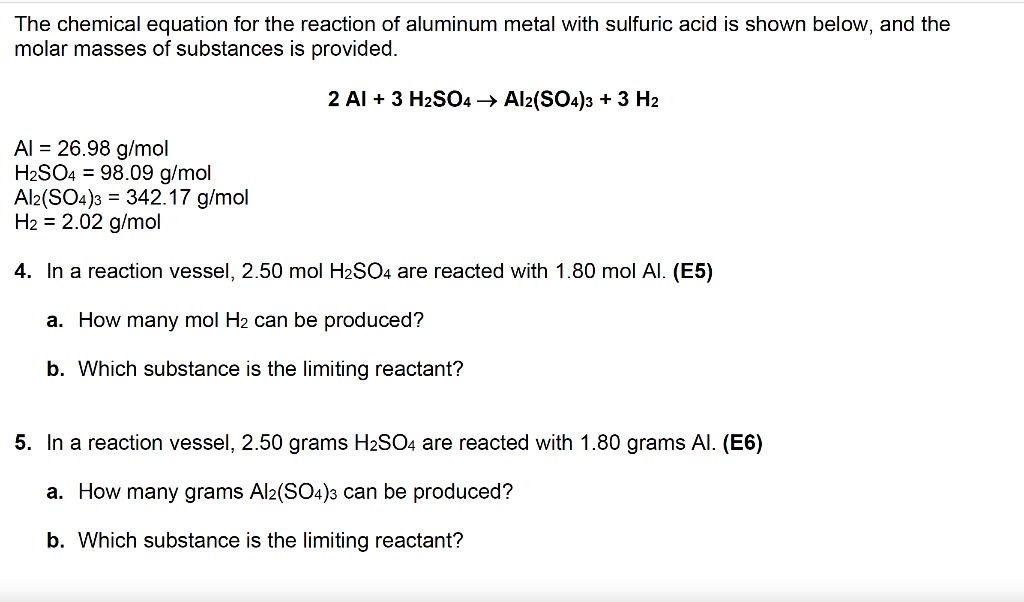
Alumminum hydroxide reacts with sulfuric acid as follows: 2Al(OH)3+H2SO4-->Al2(SO4)+6H2O. Which reagent is the limiting reactant when 0.500 mol Al(OH)3 and 0.500 mol H2SO4 are allowed to react? How ma | Homework.Study.com
100 ml of 3 mol H2SO4 reacts with 100 ml of 3 mol NaOH . What is the enthalpy of neutralisation of reaction?
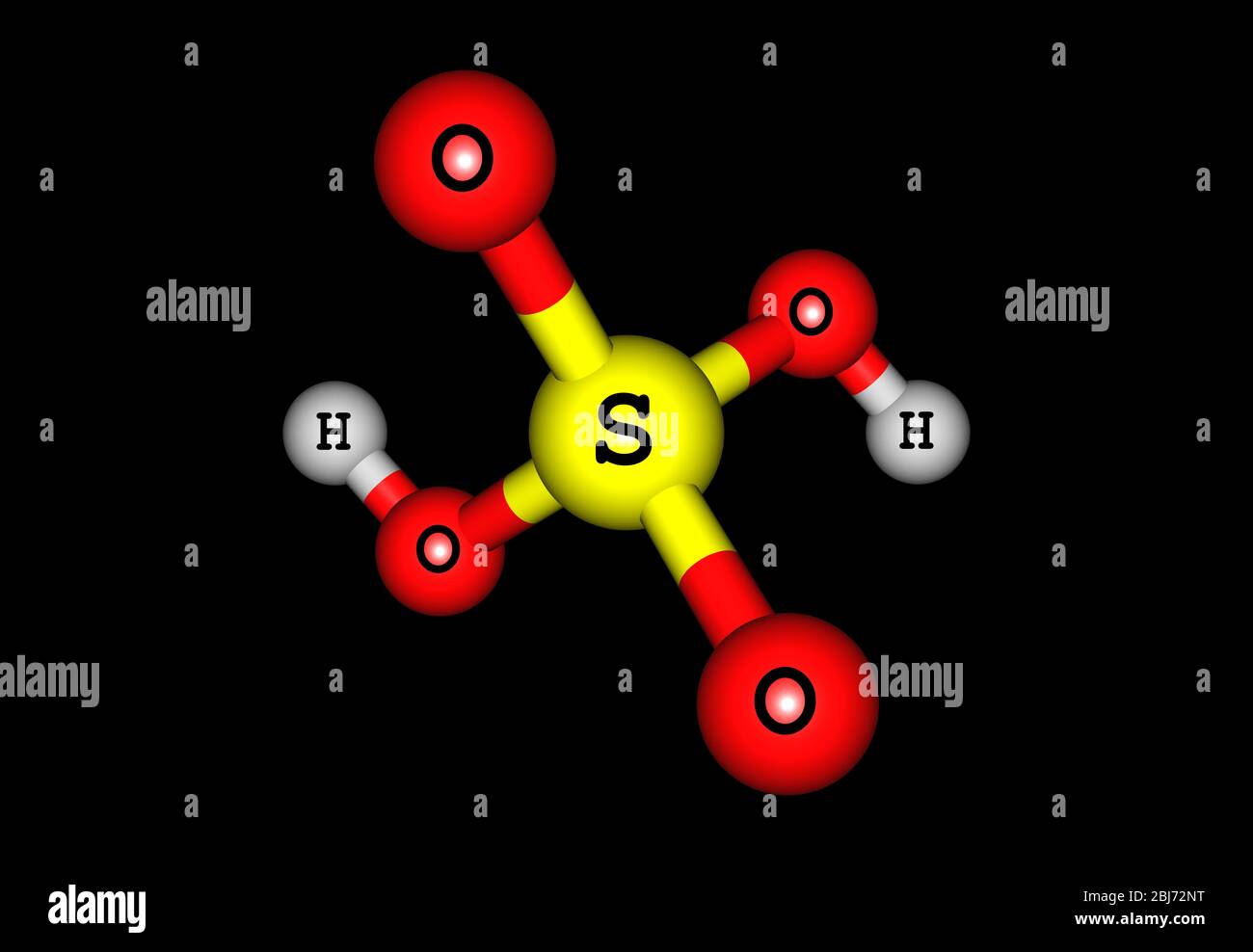
Sulfuric acid (sulphuric acid) is a highly corrosive strong mineral acid with the molecular formula H2SO4. It is a pungent-ethereal, colorless to slig Stock Photo - Alamy
Gas permeance change of each substrate of 1 mol L −1 H2SO4 immersion... | Download Scientific Diagram





![Sulfuric Acid 94+% [H2SO4] [CAS_7664-93-9] (750 Lb Drum) – Wintersun Sulfuric Acid 94+% [H2SO4] [CAS_7664-93-9] (750 Lb Drum) – Wintersun](https://cdn.shopify.com/s/files/1/0724/7981/products/19-091-01_b36c47b5-0995-4fd3-83e2-23c520d87df5.jpg?v=1550250752)