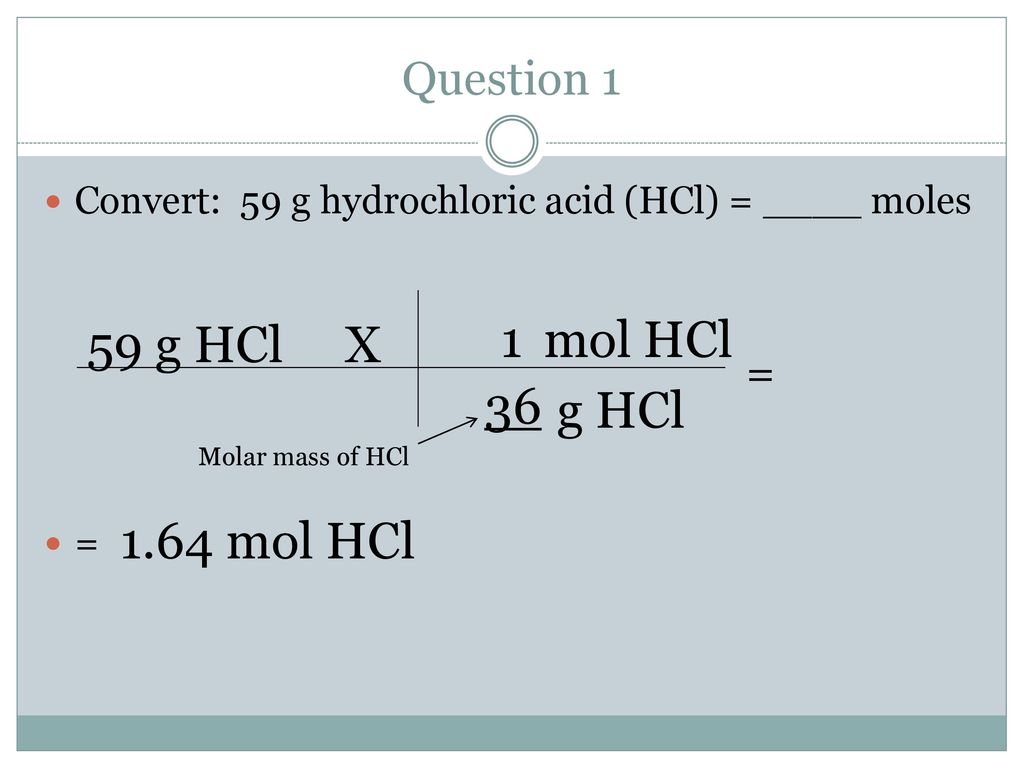
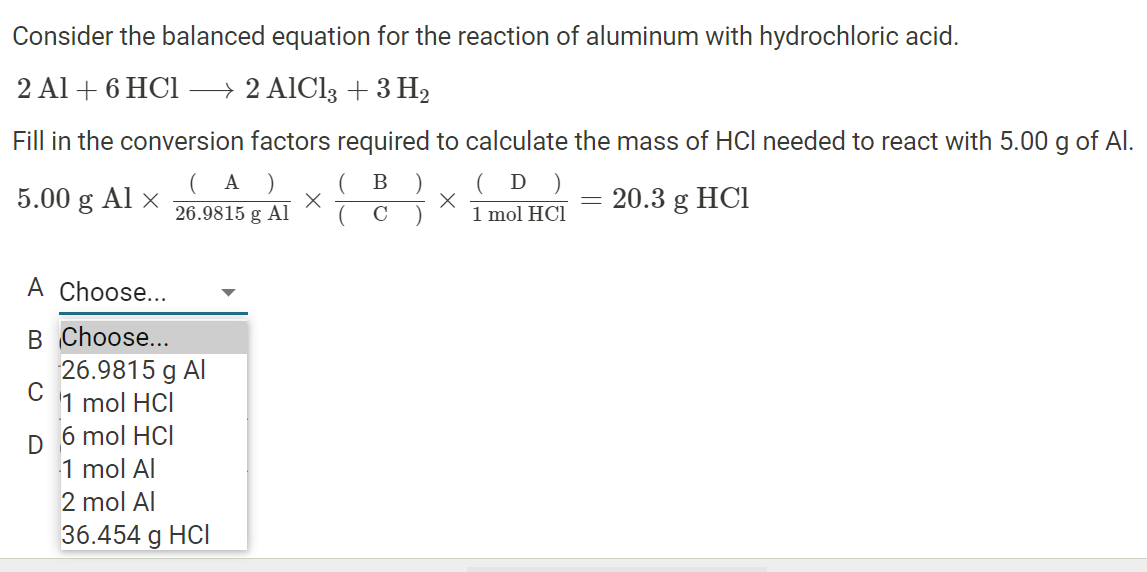
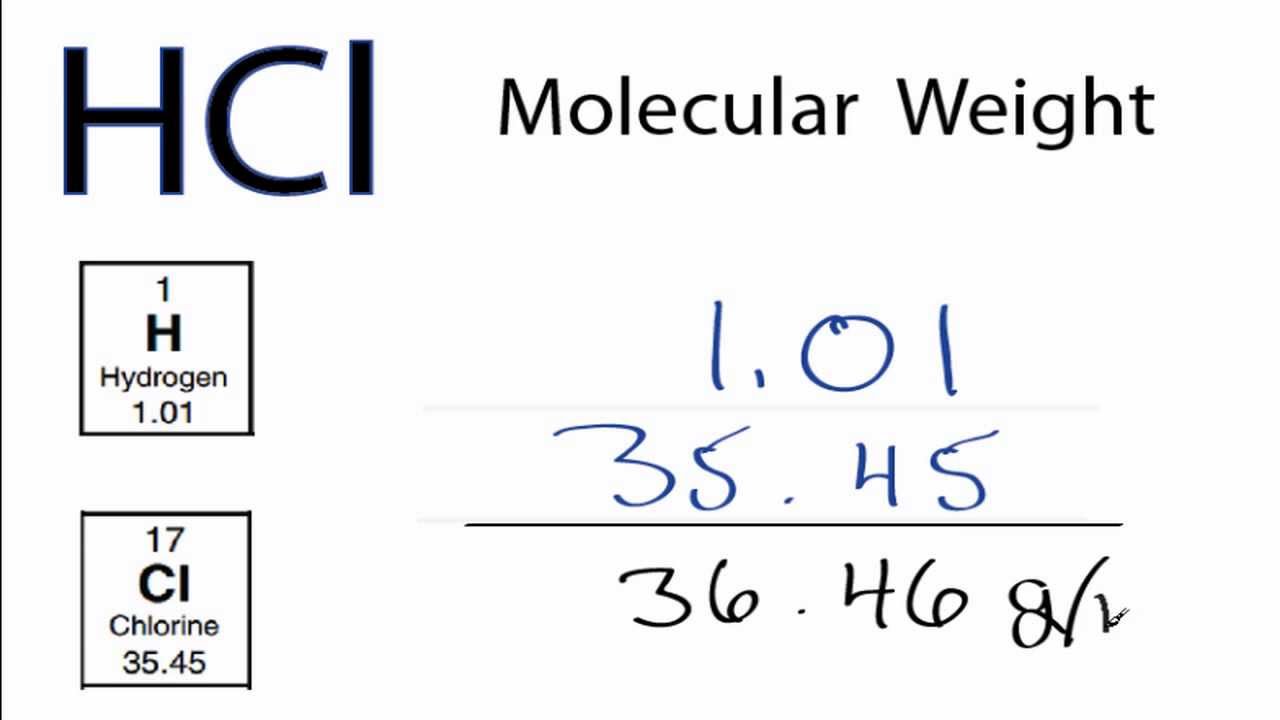Which of the following combinations will make a buffer solutions?(i) CH3COONa (2 mol) + HCl (1 mol)(ii) CH3 COOH (2 mol) + NaOH (1 mol)(ii) CH3COOH (1 mol) + CH3COONa (1 mol)(1) (
After mixing some Zn with 50 cm3. 05 M HCl, the additional HCl was neutralised by 10.75 cm3. 12 M NaOH. How much zinc was used? - Quora

25.0 mL of 1.0 M HCl is combined with 35.0 mL of 0.5 M NaOH. The initial temperature of the solutions is 25^oC , the density of the solution is 1.0 g/mL,

Polarization curves for carbon steel in 1 mol L -1 HCl in the absence... | Download Scientific Diagram
What volume of 0.1mol/dm3 hydrochloric acid will be required to neutralize 20cm3 of 2.0mol/DM3 sodium hydroxide? - Quora