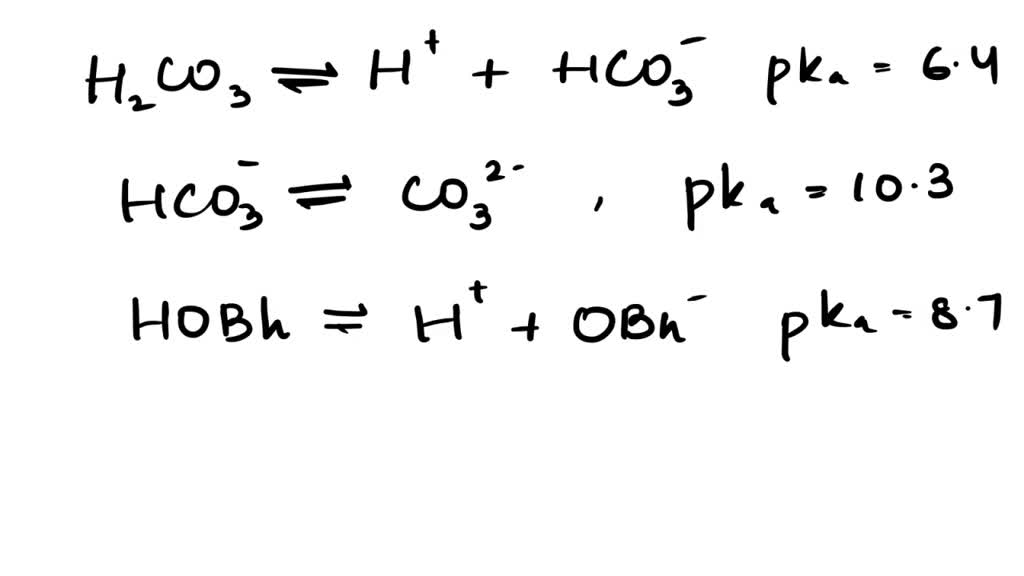
SOLVED: The pKas of H2CO3 are 6.4 10.3. The pKa of HOBr is 8.7. If equimolar amounts of Na2CO3 and HOBr are dissolved in water what will be the predominant anionic species

The pKa of a weak acid, HA, is 4.80. The pKb of a weak base, BOH, is 4.78. What is the pH of an aqueous solution of the corresponding slat BA?
A solution is prepared by mixing 0.01 mol each of H2CO3, NaHCO3, Na2CO3, and NaOH in 100 mL of water. - Sarthaks eConnect | Largest Online Education Community
![SOLVED: A buffer made from NaHCO3 and Na2CO3 is prepared with a pH of 9.40. a. What must the [CO3 ] /[HCO3 ] ratio be? Ka for HCO3 is 4.7 x 10 . SOLVED: A buffer made from NaHCO3 and Na2CO3 is prepared with a pH of 9.40. a. What must the [CO3 ] /[HCO3 ] ratio be? Ka for HCO3 is 4.7 x 10 .](https://cdn.numerade.com/previews/bd62afea-5060-4429-b9f4-4515705d9462_large.jpg)
SOLVED: A buffer made from NaHCO3 and Na2CO3 is prepared with a pH of 9.40. a. What must the [CO3 ] /[HCO3 ] ratio be? Ka for HCO3 is 4.7 x 10 .

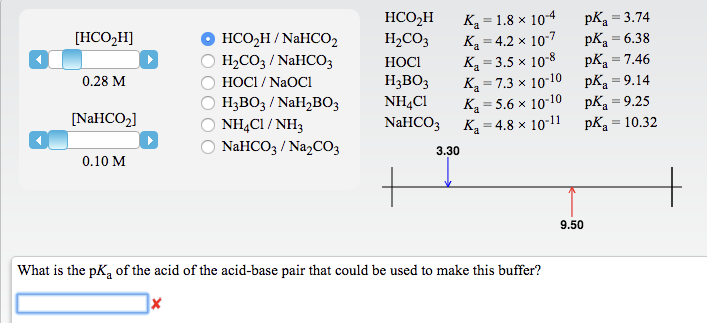
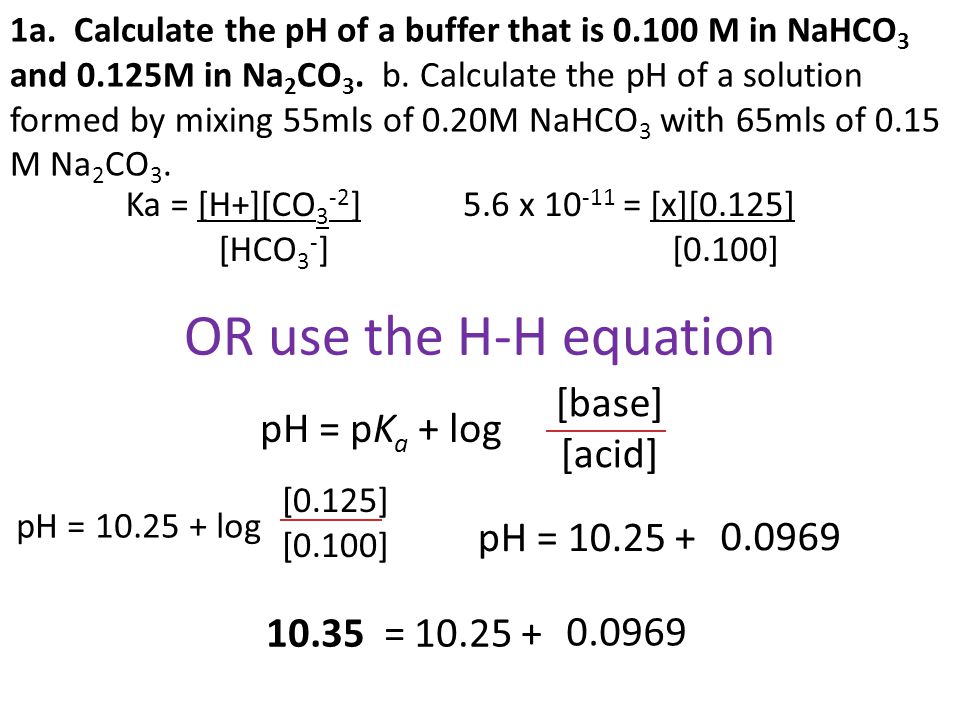
OneClass: Using the Ka values, calculate the pH of a buffer that contains the given concentrations of...
Synthesis of Unsymmetrical Diaryl Acetamides, Benzofurans, Benzophenones, and Xanthenes by Transition-Metal-Free Oxidative Cross
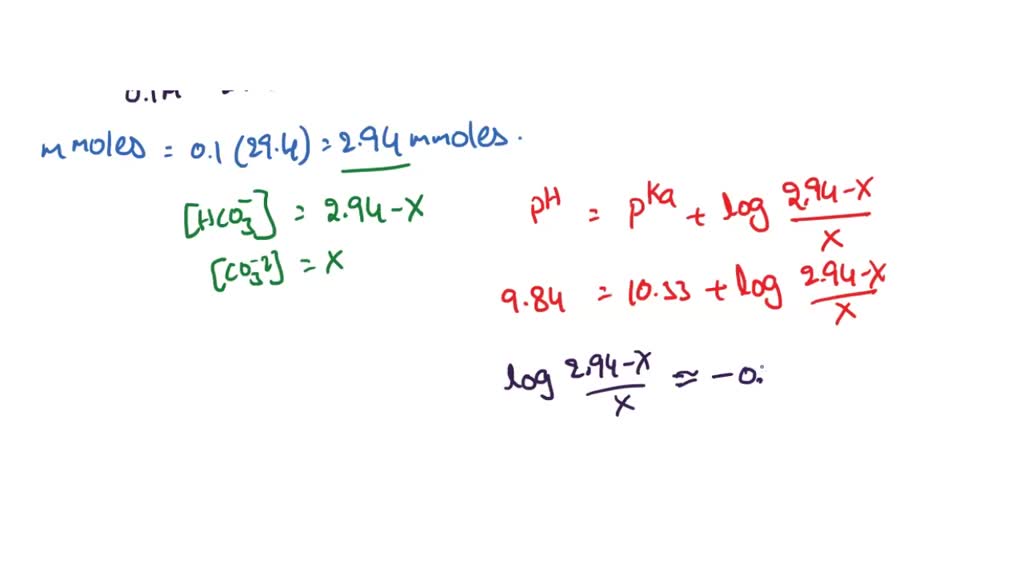
SOLVED: You are asked to prepare 500 mL of NaHCO3/Na2CO3 buffer of pH 9.87. What is the mole ratio for Na2CO3 and NaHCO3 that you are goanna mix up? For the ionization
Boehm Titration Revisited (Part I): Practical Aspects for Achieving a High Precision in Quantifying Oxygen-Containing Surface Gr
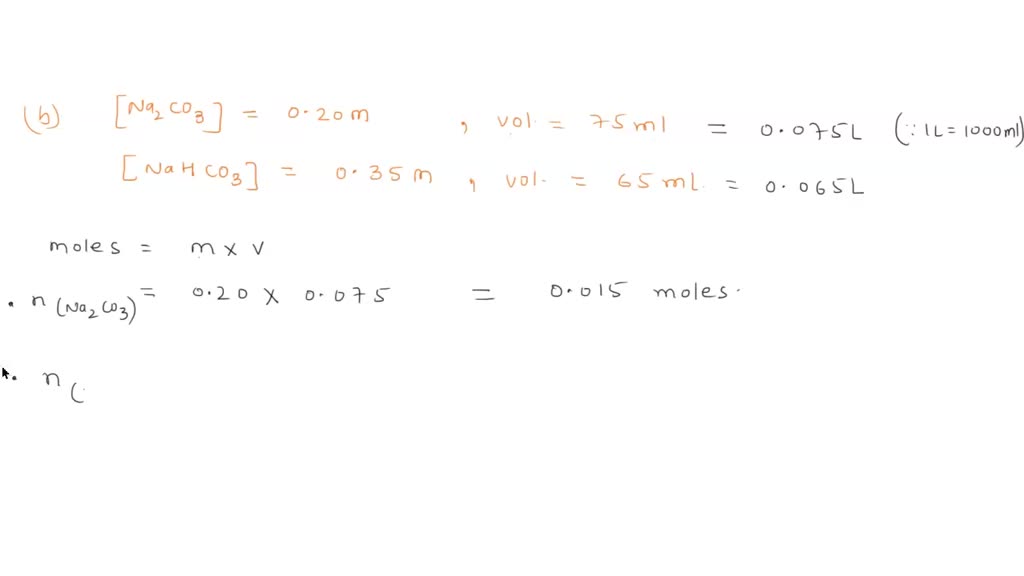
![The pKa values for various precipitants [17]. | Download Scientific Diagram The pKa values for various precipitants [17]. | Download Scientific Diagram](https://www.researchgate.net/publication/339359335/figure/tbl1/AS:860297669640196@1582122356901/The-pKa-values-for-various-precipitants-17.png)


![The pKa values for various precipitants [17]. | Download Scientific Diagram The pKa values for various precipitants [17]. | Download Scientific Diagram](https://www.researchgate.net/publication/339359335/figure/tbl1/AS:860297669640196@1582122356901/The-pKa-values-for-various-precipitants-17_Q320.jpg)